Abstract
Introduction Compounds inducing CRBN-mediated degradation of Ikaros/Aiolos have demonstrated antitumor activity in pts with NHL. The pharmacodynamic (PD) effects of these agents include T-cell activation, increased trafficking of macrophages, and increased number of regulatory T cells (Tregs) in peripheral blood (PB). Furthermore, high baseline immune infiltration in tumors has been correlated with improved outcomes.
CC-99282 (BMS-986369) is a novel CELMoD® agent that has enhanced antiproliferative, apoptotic, and immune-stimulatory effects compared with other immunomodulatory agents in various preclinical NHL models. Part A of the CC-99282-NHL-001 study (NCT03930953) showed CC-99282 monotherapy has a predictable and manageable safety profile, dominated by the class effect of neutropenia, and promising efficacy (objective response rate 42%) in heavily pretreated pts with R/R NHL.
Methods CC-99282-mediated immune effects and the dependency of response on immune changes were evaluated using biomarker data from part A of CC-99282-NHL-001, including longitudinal substrate degradation, circulating tumor DNA (ctDNA), ex vivo cytokine secretion, immunophenotyping, and baseline tumor RNA-seq data.
Results At steady state, CC-99282 induced deep (> 90%) and sustained degradation of Ikaros/Aiolos in monocytes, B cells, and T cells that was independent of response. PD effects were also confirmed in tumor cells obtained at screening and on treatment. PB T cells from pts dosed with CC-99282 showed increased secretion of IL-2, TNFα, INFγ, and GM-CSF on ex vivo stimulation with anti-CD3.
Tumor and PB biomarkers were analyzed for correlation to response. ctDNA data available for 39/50 pts treated in part A showed high tumor burden (> 2.5 log10 of tumor-specific mutant sequence [Kurtz, et al. J Clin Oncol 2018]) in 28/39 pts (72%) at study entry, and yet 13/28 pts (43%) still achieved a partial or complete response. B-cell content estimation in screening biopsies, calculated from bulk RNA-seq data using cell-type-specific markers (Bindea, et al. Immunity 2013), confirmed similar B-cell content in responders and non-responders prior to CC-99282 treatment. In contrast to treatment with other CELMoD agents, this analysis showed that durable responses to CC-99282 were observed in pts with R/R NHL despite their high tumor burden and low immune infiltration.
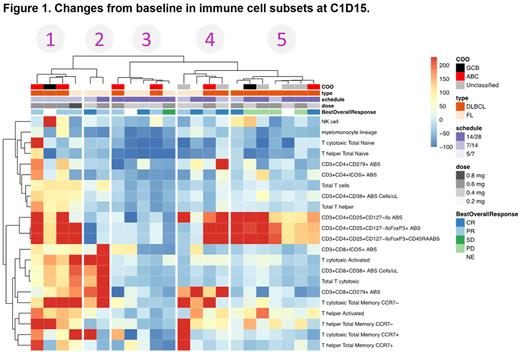
Baseline PB immunophenotype was not significantly associated with response. However, unsupervised clustering of changes in immune cell subsets in pts between baseline and cycle (C) 1 day (D) 15, revealed 5 clusters (Figure 1). Cluster 5, enriched in diffuse large B-cell lymphoma (DLBCL) non-responders, showed upregulation of peripheral Tregs without changes in T-cell activation. Clusters 1 and 4 also showed upregulation of Tregs, with cluster 1 showing additional immune activation. Cluster 2, enriched for follicular lymphoma (FL), only showed T-cell activation. Cluster 3 was enriched in responders without changes in immune subsets upon CC-99282 treatment, suggesting a stronger direct effect on tumor cells in the early stages of treatment. Baseline tumor biopsies from pts in all clusters had similar Treg levels, independent of their immune changes upon treatment. Further, longitudinal analysis of ctDNA during treatment showed that all clusters of responders had a similar decrease in ctDNA that was maintained until the end of treatment despite increases in Tregs.
Immunophenotyping at C2D1 and C3D1 showed that immune activation was maintained in responders and increases in Tregs progressively extended to all pts independently of response. The implications of these differences remain unclear. The only previous systematic analyses of the prognostic role of Tregs in lymphoma concluded that increase in Tregs correlated with longer overall survival for pts with DLBCL, but found no significant association in pts with FL (Glowala-Kosinska, et al. Eur J Haematol 2013; Peng, et al. J Cancer Res Clin Oncol 2020).
Conclusion Taken together, these results indicate a link between patterns of immune activation and response to CC-99282, but whether this link is causative, or correlative, remains to be elucidated. Responder subsets were identified based on effects on Tregs and immune activation; however, all cases led to durable responses. Further study and validation of these data could provide rationale for pt selection and combination strategies.
Disclosures
Carrancio:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Chou:Bristol Myers Squibb: Current Employment; Takeda: Ended employment in the past 24 months. Risueño:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Guarinos:Bristol Myers Squibb: Current Employment. Lopez:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Michot:Bristol Myers Squibb: Other: Nonfinancial support, support for travel to and accomodation at EHA 2022 to present CC-99282-NHL-001 study data;, Research Funding; AstraZeneca: Other: Nonfinancial support, Research Funding; Boehringer Ingelheim: Other: Nonfinancial support, Research Funding; GlaxoSmithKline: Other: Nonfinancial support, Research Funding; MedImmune: Other: Nonfinancial support; INCa: Research Funding; Janssen Cilag: Research Funding; Merck: Other: Nonfinancial support, Research Funding; Pfizer: Other: Nonfinancial support, Research Funding; Roche: Other: Nonfinancial support, Research Funding; Sanofi: Research Funding; NH TherAGUuiX: Other: Nonfinancial support. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding; Genentech/Roche, MEI, Takeda: Other: DSMC. Chavez:Janssen: Research Funding; Merck: Research Funding; TG Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Honoraria; Astrazeneca: Research Funding; ADC Therapeutics: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epyzime: Honoraria; AdiCet: Consultancy; GenMab: Consultancy. Carpio:Gilead: Honoraria; Takeda: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Honoraria; Astrazeneca: Honoraria. Feldman:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene Corporation: Speakers Bureau; Corvus Pharmaceuticals, Inc.: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Biotech, Inc.: Speakers Bureau; Juno: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kymera: Research Funding; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC: Speakers Bureau; Portola: Research Funding; Poteligeo: Speakers Bureau; SecuraBIO: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics, Inc.: Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Tessa: Research Funding. Morillo Giles:Janssen: Honoraria; Abbvie: Honoraria; Takeda: Honoraria. Pinto:Servier Affaires Medicales: Honoraria; Merck Sharp and Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche AG, Merck Sharp and Dohme, Incyte (Italy): Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche AG, Incyte (Italy), Merck Sharp and Dohme, Servier Affaires Medicales: Honoraria. Greenawalt:Bristol Myers Squibb: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties: Biomarker patents held by BMS. Pierce:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Pourdehnad:Bristol Myers Squibb: Current Employment, Patents & Royalties: Patents owned by BMS.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal